Pharmacogenomics Integration, Trial Outsourcing, and Real-Time Monitoring Boosting UK Clinical Trials Market Size Expansion
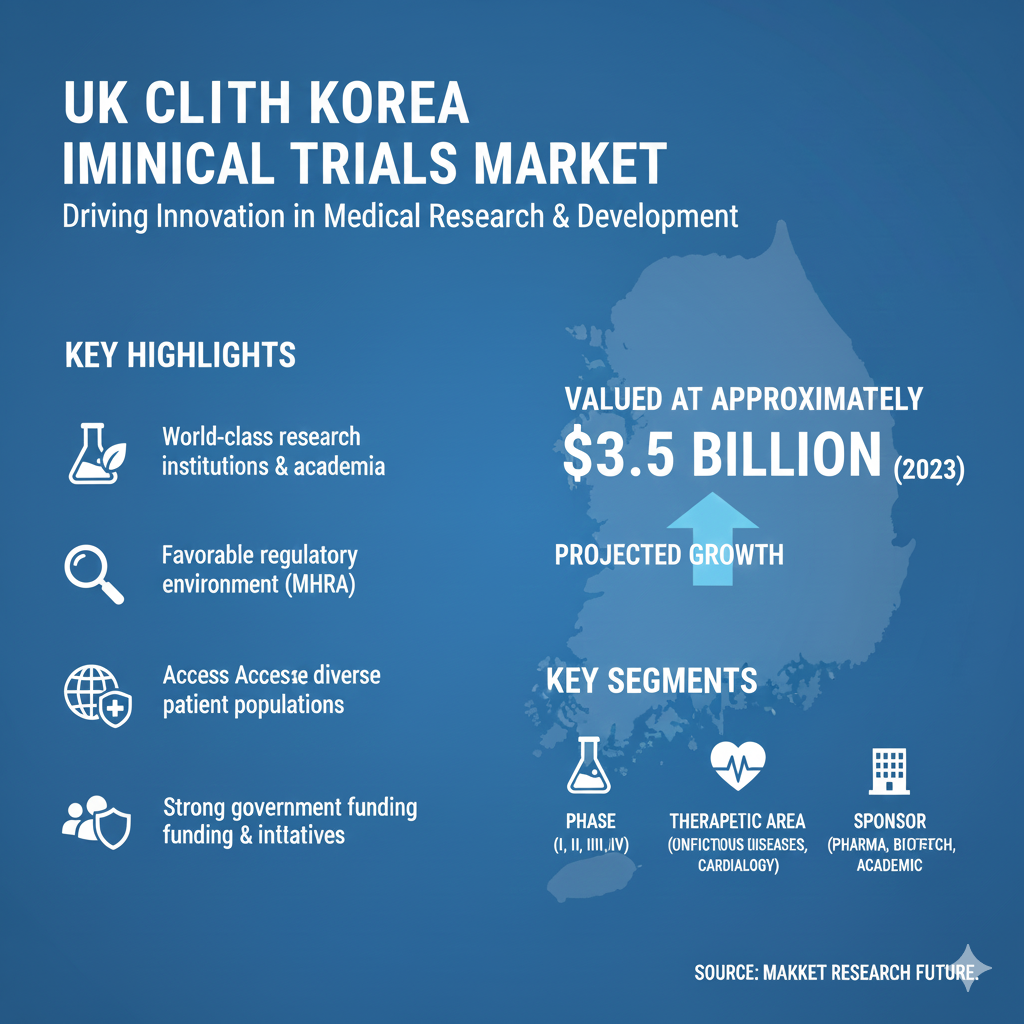
The UK Clinical Trials Market is expanding as pharmacogenomic-enabled drug trials increase investment in personalized medicine. Genomic testing helps determine individual drug response patterns, leading to targeted therapies and optimized dosing models. Clinical outsourcing among UK hospitals and contract research organizations (CROs) also strengthens trial execution. These operational and technological drivers underpin the growing outcomes highlighted in UK Clinical Trials Market Size studies across major therapeutic sectors.
Integrated real-time monitoring systems allow pharmaceutical sponsors to observe adverse effects early and make adaptive modifications. CRO partnerships streamline data workflows and regulatory documentation, reducing operational complexities associated with multi-location research. The trend toward outsourcing clinical and statistical services enables large pharma firms to accelerate trial timelines.
Growing demand for cell therapies, mRNA vaccines, biosimilars, and antibody-based treatments is driving infrastructure expansion across UK facilities. Continued investment in biomanufacturing and laboratory digitalization is increasing capacity for complex, high-precision trial designs. This market growth is supported by initiatives focusing on interoperability and patient safety monitoring.
FAQs
Q1. How does pharmacogenomics support clinical expansion?
By customizing drug dosing and improving treatment response outcomes.
Q2. Why is outsourcing essential for clinical scaling?
It reduces time, lowers cost, and provides access to specialized expertise.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


